Unit 2 :- Properties of pure Substance & Steam
1) What is the saturated solid state?
a. a state at which solid can change into liquid at constant pressure but changing temperature
b. a state at which solid can change into liquid at constant temperature but change in pressure
c. a state at which solid can change into liquid at constant pressure and temperature
d. none of the above
a. a state at which solid can change into liquid at constant pressure but changing temperature
b. a state at which solid can change into liquid at constant temperature but change in pressure
c. a state at which solid can change into liquid at constant pressure and temperature
d. none of the above
ANSWER: a state at which solid can change into liquid at constant pressure and temperature
2) What is a liquid, whose temperature is less than the saturation temperature at the given pressure, called?
a. compressed liquid
b. subcooled liquid
c. both a. and b.
d. none of the above
a. compressed liquid
b. subcooled liquid
c. both a. and b.
d. none of the above
ANSWER: both a. and b.
3) What is the degree of subcooling?
a. the difference between saturation temperature of liquid and actual temperature of liquid
b. the difference between saturation temperature of vapour and actual temperature of liquid
c. the difference between saturation temperature of liquid and actual temperature of vapour
d. the difference between saturation temperature of vapour and actual temperature of vapour
a. the difference between saturation temperature of liquid and actual temperature of liquid
b. the difference between saturation temperature of vapour and actual temperature of liquid
c. the difference between saturation temperature of liquid and actual temperature of vapour
d. the difference between saturation temperature of vapour and actual temperature of vapour
ANSWER: the difference between saturation temperature of liquid and actual temperature of liquid
4) The dryness (x) fraction of superheated steam is taken as
a. x= 0
b. x= 0.9
c. x= 0.999
d. x= 1
a. x= 0
b. x= 0.9
c. x= 0.999
d. x= 1
ANSWER: x= 1
5) What is the line which starts from critical point having constant dryness fraction throughout the line as shown in figure (Line1) called?
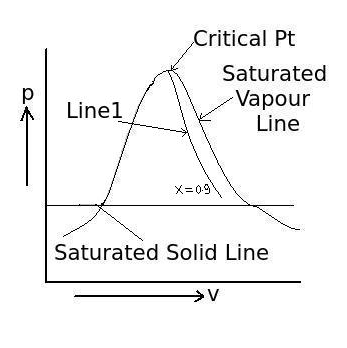 a. constant vapour line
a. constant vapour line
b. constant liquid line
c. constant quality line
d. none of the above
b. constant liquid line
c. constant quality line
d. none of the above
ANSWER: constant quality line
6) What is the dryness fraction (x) on saturated vapour line?
a. x = 0
b. x = 1
c. x = 0.9
d. x = 0.5
a. x = 0
b. x = 1
c. x = 0.9
d. x = 0.5
ANSWER: x = 1
7) What is the dryness fraction (x) for saturated water, when water just starts boiling?
a. x = 0
b. x = 1
c. x = 0.9
d. x = 0.5
a. x = 0
b. x = 1
c. x = 0.9
d. x = 0.5
ANSWER: x = 0
8) If m1 and m2 are the masses of liquid and vapour respectively in a liquid-vapour mixture, then what is the formula for dryness fraction x?
a. x = (m1 + m2) / m1
b. x = (m1 + m2) / m2
c. x = m1 / (m1 + m2)
d. x = m2 / (m1 + m2)
a. x = (m1 + m2) / m1
b. x = (m1 + m2) / m2
c. x = m1 / (m1 + m2)
d. x = m2 / (m1 + m2)
ANSWER: x = m2 / (m1 + m2)
9) The temperature at which a pure liquid transforms into vapour at constant pressure is called as
a. vaporisation temperature
b. normal temperature
c. saturation temperature
d. none of the above
a. vaporisation temperature
b. normal temperature
c. saturation temperature
d. none of the above
ANSWER: saturation temperature
10) The temperature of a substance at which the vapour pressure is equal to 760 mm Hg is called as
a. normal vapour point
b. normal boiling point
c. normal pressure point
d. none of the above
a. normal vapour point
b. normal boiling point
c. normal pressure point
d. none of the above
ANSWER: normal boiling point
11) What is the area highlighted between the saturated solid line and the saturated liquid line with respect to solidification in the following p-v diagram of pure substances called?
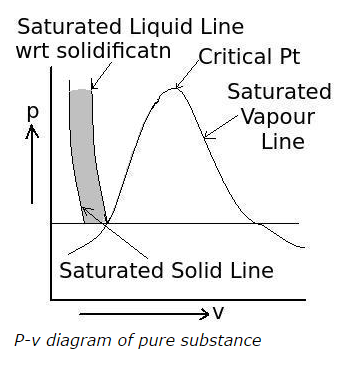 a. solid region
a. solid region
b. solidified liquid region
c. solid-liquid mixture region
d. liquid region
b. solidified liquid region
c. solid-liquid mixture region
d. liquid region
ANSWER: solid-liquid mixture region
12) What is the area highlighted between the two saturated liquid lines in the following p-v diagram of pure substance called?
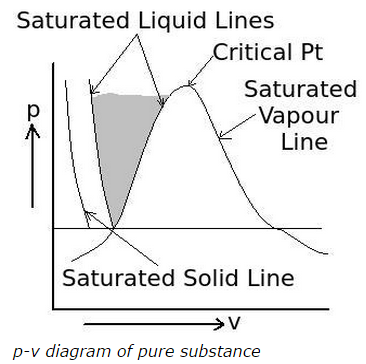 a. saturated liquid region
a. saturated liquid region
b. compressed liquid region
c. unsaturated solid region
d. solid-liquid region
b. compressed liquid region
c. unsaturated solid region
d. solid-liquid region
ANSWER: compressed liquid region
13) What is the state, at which saturated liquid line with respect to vaporisation and saturated vapour line on p-v diagram of pure substance, meet called?
a. saturation state
b. critical state
c. vaporisation state
d. superheated vapour state
c. vaporisation state
d. superheated vapour state
ANSWER: critical state
14) Which is a state of a substance from which a phase change occurs without a change of pressure or temperature?
a. pure state
b. phase state
c. saturation state
d. critical state
a. pure state
b. phase state
c. saturation state
d. critical state
ANSWER: saturation state
15) Which in the examples is NOT a pure substance?
a. baking soda
b. diamond
c. sea water
d. table salt
a. baking soda
b. diamond
c. sea water
d. table salt
ANSWER: sea water
16) A pure substance exists in
a. solid phase
b. liquid phase
c. gaseous phase
d. all of the above
a. solid phase
b. liquid phase
c. gaseous phase
d. all of the above
ANSWER: all of the above
17) What is a pure substance?
a. a homogeneous mixture of two substances of same composition
b. a substance with constant chemical composition throughout its mass
c. both a. and b.
d. none of the above
a. a homogeneous mixture of two substances of same composition
b. a substance with constant chemical composition throughout its mass
c. both a. and b.
d. none of the above
ANSWER: a substance with constant chemical composition throughout its mass
18) Which of the following represents the specific volume during phase transition.
a) Vf-Vgb) Vg-Vf
c) Vf+Vg
d) none of the mentioned
Answer: b
Explanation: Here Vg is the specific volume of the saturated vapour and Vf is the specific volume of the saturated liquid.
Explanation: Here Vg is the specific volume of the saturated vapour and Vf is the specific volume of the saturated liquid.
19). At critical point, value of Vg-Vf is
a) two
b) one
c) zero
d) infinity
a) two
b) one
c) zero
d) infinity
Answer: c
Explanation: As pressure increases, there is a decrease in Vg-Vf and at critical point its value becomes zero.
Explanation: As pressure increases, there is a decrease in Vg-Vf and at critical point its value becomes zero.
20). Above the critical point, the isotherms are continuous curves.
a) true
b) false
a) true
b) false
Answer: a
Explanation: These continuous curves approach equilateral hyperbolas at large volumes and low pressures.
Explanation: These continuous curves approach equilateral hyperbolas at large volumes and low pressures.
21). A rigid tank contains 50 kg of saturated liquid water at 90°C. Determine the pressure in the tank and the volume of the tank.
a) 0.0518 m3
b) 0.0618 m3
c) 0.0718 m3
d) 0.0818 m3
a) 0.0518 m3
b) 0.0618 m3
c) 0.0718 m3
d) 0.0818 m3
Answer: a
Explanation: P = Psat@90 C = 70.183 kPa
v = vf@90 C = 0.001036 m3/kgTotal volume of the tank = mv = (50kg)( 0.001036 m3/kg)
= 0.0518 m3.
22). A piston –cylinder device contains 0.06m3 of saturated water vapour at 350 kPa pressure. Determine the temperature and mass of the vapour inside the cylinder.
a) 0.104 kgb) 0.124 kg
c) 0.134 kg
d) 0.114 kg
Answer: d
Explanation: T = Tsat@350kPa = 138.86°C
v = vg@350kPa = 0.52422 m3/kg
m = V/v = 0.06 m3/0.52422 m3/kg = 0.114 kg.
Explanation: T = Tsat@350kPa = 138.86°C
v = vg@350kPa = 0.52422 m3/kg
m = V/v = 0.06 m3/0.52422 m3/kg = 0.114 kg.
23). A rigid tank contains 10 kg of water at 90°C. If 8 kg of the water is in the liquid form and the rest is in the vapour form, determine the pressure in the tank.
a) 60.183 kPa
b) 70.183 kPa
c) 80.183 kPa
d) 90.183 kPa
a) 60.183 kPa
b) 70.183 kPa
c) 80.183 kPa
d) 90.183 kPa
Answer: b
Explanation: P = Psat@90°C = 70.183 kPa.
Explanation: P = Psat@90°C = 70.183 kPa.
24). A rigid tank contains 10 kg of water at 90°C. If 8 kg of the water is in the liquid form and the rest is in the vapour form, determine the volume of the tank.
a) 1.73 m3
b) 2.73 m3
c) 3.73 m3
d) 4.73 m3
b) 2.73 m3
c) 3.73 m3
d) 4.73 m3
Answer: d
Explanation: P = Psat@90°C = 70.183 kPa@ 90°C, vf = 0.001036 m3/kg and vg = 2.3593 m3/kg
V = Vf + Vg = mf vf + mg vg = 4.73 m3.
25). An 80 litre vessel contains 4 kg of R-134a at a pressure of 160 kPa. Determine the temperature.
a) -10.60°C
b) -13.60°C
c) -15.60°C
d) -19.60°C
a) -10.60°C
b) -13.60°C
c) -15.60°C
d) -19.60°C
Answer: c
Explanation: v = V/m = 0.080 m3/4 kg = 0.02 m3/kg
@ 160kPa, vf = 0.0007437 m3/kg; vg = 0.12348 m3/kg
vf < v < vg Therefore T = Tsat@160kPa = -15.60°C.
Explanation: v = V/m = 0.080 m3/4 kg = 0.02 m3/kg
@ 160kPa, vf = 0.0007437 m3/kg; vg = 0.12348 m3/kg
vf < v < vg Therefore T = Tsat@160kPa = -15.60°C.
26). An 80 litre vessel contains 4 kg of R-134a at a pressure of 160 kPa. Determine the quality.
a) 0.127
b) 0.137
c) 0.147
d) 0.157
a) 0.127
b) 0.137
c) 0.147
d) 0.157
Answer: d
Explanation: v = V/m = 0.080 m3/4 kg = 0.02 m3/kg
@ 160kPa, vf = 0.0007437 m3/kg; vg = 0.12348 m3/kg.
vf < v < vg
x = (v –vf)/ vfg = 0.157.
Explanation: v = V/m = 0.080 m3/4 kg = 0.02 m3/kg
@ 160kPa, vf = 0.0007437 m3/kg; vg = 0.12348 m3/kg.
vf < v < vg
x = (v –vf)/ vfg = 0.157.
27). An 80 litre vessel contains 4 kg of R-134a at a pressure of 160 kPa. Determine the volume occupied by the vapour phase.
a) 0.0775 m3
b) 0.0575 m3
c) 0.0975 m3
d) 0.0375 m3
a) 0.0775 m3
b) 0.0575 m3
c) 0.0975 m3
d) 0.0375 m3
Answer: a
Explanation: v = V/m = 0.080 m3/4 kg = 0.02 m3/kg@ 160kPa, vf = 0.0007437 m3/kg; vg = 0.12348 m3/kg
vf < v < vg
x = (v –vf)/ vfg = 0.157
mg = x*m(total) = 0.628kg
Vg = mg*vg = 0.0775 m3 or 77.5 litre.
28). Determine the specific volume of R-134a at 1 MPa and 50°C, using ideal gas equation of state.
a) 0.022325 m3/kg
b) 0.024325 m3/kg
c) 0.025325 m3/kg
d) 0.026325 m3/kg
a) 0.022325 m3/kg
b) 0.024325 m3/kg
c) 0.025325 m3/kg
d) 0.026325 m3/kg
Answer: b
Explanation: v = RT/P = (0.0815 kJ/kg.K)* (323 K)/(1000 kPa)
= 0.026325 m3/kg.
Explanation: v = RT/P = (0.0815 kJ/kg.K)* (323 K)/(1000 kPa)
= 0.026325 m3/kg.
29). The properties of water are arranged in the steam tables as functions of
a) pressure
b) temperature
c) pressure and temperature
d) none of the mentioned
a) pressure
b) temperature
c) pressure and temperature
d) none of the mentioned
Answer: c
Explanation: The properties of water are arranged in steam tables as functions of both pressure and temperature.
Explanation: The properties of water are arranged in steam tables as functions of both pressure and temperature.
30). The internal energy of saturated water at the triple point is
a) 1
b) 0
c) -1
d) infinity
a) 1
b) 0
c) -1
d) infinity
Answer: b
Explanation: This value is arbitrarily chosen.
Explanation: This value is arbitrarily chosen.
31). The entropy of saturated water is chosen to be zero at triple point.
a) true
b) false
a) true
b) false
Answer: a
Explanation: This is arbitrarily chosen and form the basic assumptions for steam tables.
Explanation: This is arbitrarily chosen and form the basic assumptions for steam tables.
32). When a liquid and its vapour are in equilibrium at a certain pressure and temperature, then which of the following is required to identify the saturation state.
a) pressure
b) temperature
c) both pressure and temperature
d) pressure or temperature
a) pressure
b) temperature
c) both pressure and temperature
d) pressure or temperature
Answer: d
Explanation: If one of the quantity is given, then other gets fixed.
Explanation: If one of the quantity is given, then other gets fixed.
33). Saturated liquid or the saturated vapour has how many independent variables?
a) one
b) two
c) three
d) none of the mentioned
a) one
b) two
c) three
d) none of the mentioned
Answer: a
Explanation: Only one property is required to be known to fix up the state.
Explanation: Only one property is required to be known to fix up the state.
34). If data are required for intermediate temperatures or pressures, linear interpolation is normally accurate.
a) true
b) false
a) true
b) false
Answer: a
Explanation: To reduce the amount of interpolation required, two tables are provided.
Explanation: To reduce the amount of interpolation required, two tables are provided.
35). For a liquid-vapour mixture, which of the following can give us all the properties of the mixture?
a) p or t and the quality of the mixture are given
b) p or t and any one of the property is given
c) both of the mentioned
d) none of the mentioned
a) p or t and the quality of the mixture are given
b) p or t and any one of the property is given
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: In first case, properties can be directly evaluated and in second case we can find the quality first and then evaluate all other properties.
Explanation: In first case, properties can be directly evaluated and in second case we can find the quality first and then evaluate all other properties.
36). When does a vapour become superheated?
a) when the temperature of vapour is less than the saturation temperature at given pressure
b) when the temperature of vapour is more than the saturation temperature at given pressure
c) when the temperature of vapour is equal to the saturation temperature at given pressure
d) none of the mentioned
a) when the temperature of vapour is less than the saturation temperature at given pressure
b) when the temperature of vapour is more than the saturation temperature at given pressure
c) when the temperature of vapour is equal to the saturation temperature at given pressure
d) none of the mentioned
Answer: b
Explanation: For a superheated vapour, temperature of vapour must be greater than the saturation temperature.
Explanation: For a superheated vapour, temperature of vapour must be greater than the saturation temperature.
37). The superheat or degree of superheat is given by
a) difference between the temperature of saturated liquid and saturation temperature
b) difference between the temperature of superheated vapour and saturation temperature
c) sum of the temperature of superheated vapour and saturation temperature
d) none of the mentioned
a) difference between the temperature of saturated liquid and saturation temperature
b) difference between the temperature of superheated vapour and saturation temperature
c) sum of the temperature of superheated vapour and saturation temperature
d) none of the mentioned
Answer: b
Explanation: Superheat= T1(temperature of superheated vapour) – T(saturated).
Explanation: Superheat= T1(temperature of superheated vapour) – T(saturated).
38). When the temperature of a liquid is less than the saturation temperature at the given pressure, the liquid is called compressed liquid.
a) true
b) false
a) true
b) false
Answer: a
Explanation: For a compressed liquid, temperature of liquid must be less than the saturation temperature.
Explanation: For a compressed liquid, temperature of liquid must be less than the saturation temperature.
39). The properties of liquid _____ with pressure.
a) do not vary
b) vary largely
c) vary little
d) none of the mentioned
a) do not vary
b) vary largely
c) vary little
d) none of the mentioned
Answer: c
Explanation: This is the reason why properties are taken from the saturation tables at the temperature of the compressed liquid.
40). Which of the following statement is true?
a) a subcooled liquid is one which is cooled below its saturation temperature at a certain pressure
b) subcooling is the difference between the saturation temperature and the actual liquid temperature
c) both of the mentioned
d) none of the mentioned
a) a subcooled liquid is one which is cooled below its saturation temperature at a certain pressure
b) subcooling is the difference between the saturation temperature and the actual liquid temperature
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: This is what a subcooled liquid means.
Explanation: This is what a subcooled liquid means.






No comments:
Post a Comment