Unit 1: Basic Laws of Thermodynamics
Thermodynamics Questions and Answers – First Law for a Closed System
1. Energy has different forms which include
a) heat
b) work
c) all of the mentioned
d) none of the mentioned
Answer: c
Explanation: Basic fact about energy.
2. Work input is directly proportional to heat and the constant of proportionality is called
a) joule’s equivalent
b) mechanical equivalent of heat
c) all of the mentioned
d) none of the mentioned
Answer: c
Explanation: True for a closed system undergoing a cycle.
3. The value of constant of proportionality, J, has the value
a) 1
b) 0
c) -1
d) infinity
Answer: a
Explanation: In the S.I. system, both heat and work are measured in the derived unit of energy, the Joule.
4. It was Joule who first established that heat is a form of energy, and thus laid the foundation of the first law of thermodynamics.
a) true
b) false
Answer: a
Explanation: Prior to Joule, heat was considered to be an invisible fluid flowing from a body of higher calorie to a body of lower calorie.
5. Which of the following represents the energy in storage?
a) heat
b) work
c) internal energy
d) none of the mentioned
Answer: c
Explanation: Energy in storage is internal energy or the energy of the system.
6. By first law of thermodynamics,
a) Q=ΔE-W
b) Q=ΔE+W
c) Q=-ΔE-W
d) Q=-ΔE+W
Answer: b
Explanation: Q-W is the net energy stored in system and is called internal energy of system.
Thermodynamics Questions and Answers – Specific Heat at Constant Volume and Pressure and Control Volume
1. The specific heat of a substance at constant volume is defined as the rate of change of ___ with respect to ___
a) specific internal energy, temperature
b) work, pressure
c) specific internal energy, pressure
d) heat, temperature
Answer: a
Explanation: cv=∂u/∂T at constant volume.
2. Heat transferred at constant _____ increases the _____ of a system.
a) pressure, increases
b) volume, increases
c) both of the mentioned
d) none of the mentioned
Answer: c
Explanation: At constant pressure, (dQ)=dh and at constant volume, Q=Δu.
3. Specific heat of a substance at constant volume is a property of the system.
a) true
b) false
Answer: a
Explanation: Since T,v and u are the properties of the system, specific heat at a constant volume is a property of the system.
4. The specific heat of a substance at constant pressure is defined as the rate of change of ___ with respect to ___
a) work, pressure
b) enthalpy, temperature
c) enthalpy, pressure
d) heat, temperature
Answer: b
Explanation: cp=∂h/∂T at constant pressure.
5. The heat capacity at constant pressure Cp
a) m/cp
b) cp/m
c) mcp
d) none of the mentioned
Answer: c
Explanation: Cp=(mass*specific heat at constant pressure).
6. Specific heat of a substance at constant pressure is a property of the system.
a) true
b) false
Answer: a
Explanation: cp is a property of a substance just like cv.
7. When there is mass transfer across the system boundary, the system is called
a) isolated system
b) closed system
c) open system
d) none of the mentioned
Answer: c
Explanation: Basic definition of an open system.
8. If a certain mass of steam is considered as the thermodynamic system, then the energy equation becomes
a) Q=ΔKE + ΔPE – ΔU + W
b) Q=ΔKE + ΔPE – ΔU – W
c) Q=-ΔKE – ΔPE + ΔU + W
d) Q=ΔKE + ΔPE + ΔU + W
Answer: d
Explanation: Q=ΔE + W and E=KE + PE + U.
9. The surface of the control volume is known as the control surface.
a) true
b) false
Answer: a
Explanation: This is same as the system boundary of the open system.
10. Steady flow means that the rates of flow of mass and energy across the control surface
a) varies
b) remains constant
c) depends on the control surface
d) none of the mentioned
Answer: b
Explanation: In a steady flow rate of flow remains constant.
Thermodynamics Questions and Answers – Enthalpy
a) h=u-pv
b) h=u+pv
c) h=-u+pv
d) h=-u-pv
Explanation: This is a basic definition for enthalpy.
a) heat transferred
b) work done
c) zero
d) none of the mentioned
Explanation: In a constant volume process, there is no work other than the pdV work
a) h=u-RT
b) h=-u-RT
c) h=u+RT
d) h=-u+RT
Explanation: For an ideal gas, pv=RT.
a) true
b) false
Explanation: Enthalpy is an intensive property measured mostly in kJ/kg.
a) decreases
b) increases
c) first decreases then increases
d) first increases then decreases
Explanation: At constant pressure, (dQ)=dh where h=u+pv is the specific enthalpy of the system.
a) true
b) false
Explanation: This is because the internal energy of an ideal gas depends only on the temperature.
a) H=h/m
b) H=m/h
c) H=mh
d) none of the mentioned
Explanation: Total enthalpy equals (mass*enthalpy) of substance.
a) all gases
b) steam
c) water
d) ideal gas
Explanation: The enthalpy of an ideal gas depends only on the temperature because the internal energy of an ideal gas depends only on the temperature.
a) constant volume
b) constant pressure
c) both at constant volume and pressure
d) none of the mentioned
Explanation: Change in enthalpy occurs when heat is given to a system at constant pressure.
a) pdv=d(pv)
b) dQ=du+d(pv)
c) dQ=d(u+pv)
d) all of the mentioned
Explanation: For a constant pressure process, dQ=du+pdv.
Thermodynamics Questions and Answers – Second Law of Thermodynamics
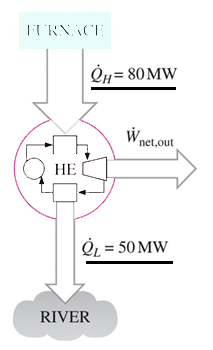
a) 30 MW
b) 40 MW
c) 50 MW
d) 60 MW
Explanation: Net power output = 80 – 50 MW = 30 MW.

a) 47.5 %
b) 27.5 %
c) 37.5 %
d) none of the mentioned
Explanation: The thermal efficiency of heat engine = net work output / heat input
= 30/80 = 0.375 = 37.5 %.
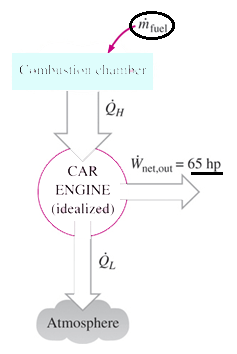
a) 0.00273 kg/s
b) 0.00373 kg/s
c) 0.00473 kg/s
d) 0.00573 kg/s
Explanation: Q = 50/0.24 = 208.3 kW,
hence fuel consumption rate = 208.3 kW / 44000 kJ/kg = 0.00473 kg/s.
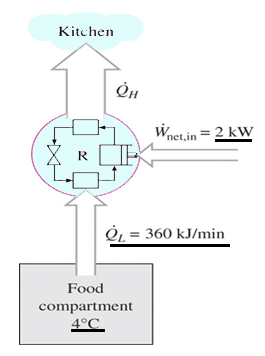
a) 4
b) 3
c) 2
d) 1
Explanation: COP = (360/2)(1/60) = 3.

a) 450 kJ/min
b) 460 kJ/min
c) 470 kJ/min
d) 480 kJ/min
Explanation: Q = 360 + (2)(60/1) = 480 kJ/min.
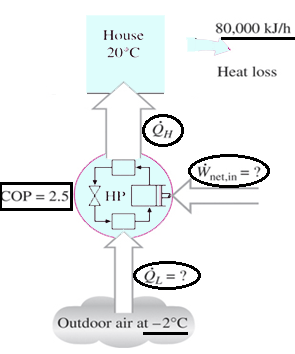
a) 32000 kJ/h
b) 33000 kJ/h
c) 34000 kJ/h
d) 35000 kJ/h
Explanation: W = Q/COP = 80000 kJ/h / 2.5 = 32000 kJ/h.

a) 32000 kJ/h
b) 48000 kJ/h
c) 54000 kJ/h
d) 72000 kJ/h
Explanation: The rate at which heat is absorbed = 80000 – 32000 = 48000 kJ/h.
a) 1.09 kW
b) 1.19 kW
c) 1.29 kW
d) 1.39 kW
Explanation: Q = m*cp*(temperature change) = 20.08 kW
COP = (15+273)/(35-15) = 14.4
hence power needed = 20/14.4 = 1.39 kW.
a) reversible
b) irreversible
c) impossible
d) none of the mentioned
Explanation: The Carnot efficiency = 1 – (400/1000) = 0.6 and real efficiency = (300/325) = 0.615 which is greater than the Carnot efficiency hence cycle is impossible.
a) 97.84 kW
b) 98.84 kW
c) 99.84 kW
d) 95.84 kW
Explanation: COP = 1.022 and thus power required = 100/1.022 = 97.84 kW.
a) 0.752 kJ
b) 0.952 kJ
c) 0.852 kJ
d) none of the mentioned
Explanation: W = thermal efficiency * Q(fuel) thus Q(fuel) = 1/(0.35*3) = 0.952 kJ.
Thermodynamics Questions and Answers – Cyclic Heat Engine
a) true
b) false
Explanation: The second law of thermodynamics provides criterion as to the probability of a process.
a) heat can be completely converted into work
b) work can be completely converted into heat
c) both heat and work are completely interchangeable
d) all of the mentioned
Explanation: Work transfer -> internal energy increase -> heat transfer.
a) work is a high grade energy
b) heat is a low grade energy
c) complete conversion of low grade energy into high grade energy in a cycle is impossible
d) all of the mentioned
Explanation: These facts are in accordance with Joule’s work and underlies the work of Carnot.
a) net heat transfer to the system and net work transfer from the system
b) net heat transfer from the system and net work transfer to the system
c) depends on the conditions of cycle
d) none of the mentioned
Explanation: This is the basic concept of cycle heat engine.
a) true
b) false
Explanation: It is an example for a cyclic heat engine
a) heat is transferred from furnace to boiler
b) work is produced in turbine rotor
c) steam is condensed in condenser
d) all of the mentioned
Explanation: These are the basic processes occurring in a heat engine cycle comprising of furnace, boiler condenser and a turbine.
a) heat input, produce work
b) produce work, heat input
c) can be both of the mentioned
d) none of the mentioned
Explanation: Net work and heat input are of primary interest in a cycle.
a) total heat output / net work input
b) total heat input / net work output
c) net work output / total heat input
d) net work input / total heat output
Explanation: Basic definition of efficiency.
a) small heat capacity
b) large heat capacity
c) infinite heat capacity
d) none of the mentioned
Explanation: Basic fact about TER.
a) true
b) false
Explanation: The changes taking place in TER are very slow and minute.
a) source, sink
b) sink, source
c) sink, sink
d) source, source
Explanation: A source transfers heat while a sink receives heat.
a) it is a large body enclosed by an adiabatic impermeable wall
b) stores work as KE or PE
c) all processes within an MER are quasi-static
d) all of the mentioned
Explanation: These are some important features of an MER.
Thermodynamics Questions and Answers – Refrigerator and Heat Pump
a) PMM1
b) PMM2
c) refrigerator
d) heat pump
Explanation: This is the main function of a refrigerator.
a) absorbs the heat leakage into body from surroundings
b) evaporates in the evaporator
c) absorbs latent heat of vaporization form the body which is cooled
d) all of the mentioned
Explanation: Refrigerant is required for the proper functioning of a refrigerator.
a) heat leakage/work input
b) work input/heat leakage
c) latent heat of condensation/work input
d) work input/latent heat of condensation
Explanation: Coefficient of performance is the performance parameter used in a refrigerator cycle.
a) PMM1
b) PMM2
c) refrigerator
d) heat pump
Explanation: This is the main function of a heat pump.
a) true
b) false
Explanation: This is just opposite to a refrigerator.
a) COP of pump=COP of refrigerator – 1
b) COP of pump=COP of refrigerator + 1
c) COP of pump=COP of refrigerator – 2
d) COP of pump=COP of refrigerator + 2
Explanation: This relation comes from the COP of pump and refrigerator.
a) true
b) false
Explanation: (Heat leakage from a heat pump to surroundings)=(COP of refrigerator + 1)*(work done on pump).
a) a heat pump provides a thermodynamic advantage over direct heating
b) COP for both refrigerator and pump cannot be infinity
c) work input for both refrigerator and pump is greater than zero
d) all of the mentioned
Explanation: W is the electrical energy used to drive the pump or refrigerator which cannot be zero.
a) not connected to each other
b) virtually two parallel statements of second law
c) violation of one doesn’t violate the other
d) none of the mentioned
Explanation: Kelvin-Planck’s and Clausius’ statements are equivalent in all aspects.
a) true
b) false
Explanation: This shows the equivalence of Kelvin-Planck’s and Clausius’ statements.
Thermodynamics Questions and Answers – Kelvin-Planck Statement and Clausius’ Statement of Second Law
a) impossible, single fixed temperature
b) possible, changing temperature
c) impossible, changing temperature
d) possible, single fixed temperature
Explanation: This is the basic definition of Kelvin-Planck statement.
a) net work=Q1 and efficiency=1.00
b) heat is exchanged only with one reservoir
c) it violates the Kelvin-Planck statement
d) all of the mentioned
Explanation: Such a heat engine is called a perpetual motion machine of the second kind(PMM2).
a) true
b) false
Explanation: A PMM2 is impossible because it violates the Kelvin-Planck statement.
a) one, one
b) one, two
c) two, two
d) none of the mentioned
Explanation: This is required to produce power.
a) true
b) false
Explanation: The first law is a separate law of nature.
a) PMM2
b) PMM1
c) PMM0
d) none of the mentioned
Explanation: This is a basic fact about PMM1.
a) heat always from a high temperature body to a low temperature body
b) heat always from a low temperature body to a high temperature body
c) heat can flow from both low to high and high to low temperature body
d) none of the mentioned
Explanation: The reverse process never occurs spontaneously.
a) it is impossible to construct a device than can transfer heat from a cooler body to a hotter body without any effect
b) it is impossible to construct a device than can transfer heat from a hotter body to a cooler body without any effect
c) it is possible to construct a device than can transfer heat from a cooler body to a hotter body without any effect
d) none of the mentioned
Explanation: To transfer heat from a cooler body to a hotter body, some work must be expended.
a) a ship could be driven by extracting heat from the ocean
b) run a power plant by extracting heat from the air
c) both of the mentioned
d) none of the mentioned
Explanation: Both of the above possibilities do not violate the first law but do violate the second law.
a) PMM0
b) PMM1
c) PMM2
d) none of the mentioned
Explanation: PMM2 violates the second law.
Thermodynamics Questions and Answers – Reversibility, Irreversibilty and causes of Irreversibilty
a) at the conclusion of process, both system and surroundings can be restored to their initial states without producing any change
b) it should not leave any trace to show that the process had ever occurred
c) it is carried out infinitely slowly
d) all of the mentioned
Explanation: These are some basic concepts of a reversible process.
a) true
b) false
Explanation: A reversible process is carried out very slowly and every state it passes through is an equilibrium state.
a) lack of equilibrium during the process
b) involvement of dissipative effects
c) both of the mentioned
d) none of the mentioned
Explanation: These two are the major causes of irreversibility.
a) infinity
b) zero
c) -1
d) 1
Explanation: For heat transfer to be reversible, heat must be transferred through an infinitesimal temperature difference.
a) irreversible
b) take place through a finite temperature difference
c) both of the mentioned
d) none of the mentioned
Explanation: An infinitesimal temperature difference is not easy to attain.
a) true
b) false
Explanation: It can be demonstrated by the second law.
a) friction, viscosity
b) inelasticity
c) electrical resistance, magnetic hysteresis
d) all of the mentioned
Explanation: These effects are known as dissipative effects.
a) PMM2
b) PMM3
c) PMM1
d) PMM0
Explanation: This is not possible since lubrication cannot be completely eliminated.
a) true
b) false
Explanation: Friction lakes the process irreversible.
a) stirring work
b) friction work in moving devices
c) current flowing through a wire
d) all of the mentioned
Explanation: All these processes includes a particular cause of irreversibility.
a) true
b) false
Explanation: This mainly includes heat interaction with the surroundings due to a finite temperature gradient.
Thermodynamics Questions and Answers – Carnot Theorem, Carnot Cycle and Reversed Heat Engine
a) true
b) false
Explanation: A reversible cycle is an ideal hypothetical cycle in which all processes are reversible.
a) 4 isothermal processes
b) 4 adiabatic processes
c) 2 isothermal and 2 adiabatic processes
d) none of the mentioned
Explanation: Two reversible isotherms and two reversible adiabatics constitute a Carnot cycle.
a) adiabatic -> adiabatic -> isothermal -> isothermal
b) adiabatic -> isothermal -> adiabatic -> isothermal
c) isothermal -> isothermal -> adiabatic -> adiabatic
d) isothermal -> adiabatic -> isothermal -> adiabatic
Explanation: Carnot cycle consists if these four processes in succession.
a) high, low, receives
b) low, high, receives
c) high, low, gives
d) low, high, gives
Explanation: In reversed heat engine, the magnitude of energy transfers remains same and only directions change.
a) heat pump
b) refrigerator
c) both of the mentioned
d) none of the mentioned
Explanation: Heat pump and refrigerator are the types of reversed heat engine.
a) true
b) false
Explanation: This is the statement of Carnot’s theorem
a) same
b) independent of the nature of working substance
c) independent of the amount of working substance
d) all of the mentioned
Explanation: This statement is a corollary of Carnot’s theorem.
a) 1-(T1/T2)
b) 1-(T2/T1)
c) (T1/T2)-1
d) (T2/T1)-1
Explanation: Efficiency=1-(Q2/Q1) and T2,T1 are temperatures at which heat is rejected and received.
a) T2/(T1-T2)
b) T1/(T1-T2)
c) T2/(T2-T1)
d) T1/(T2-T1)
Explanation: For a reversible refrigerator, (Q1/Q2)=(T1/T2).
a) T2/(T1-T2)
b) T1/(T1-T2)
c) T2/(T2-T1)
d) T1/(T2-T1)
Explanation: For a reversible heat pump we have, (Q1/Q2)=(T1/T2).






If you have any query about this blog comment us. Totalmechanicalengineering.blogspot.com
ReplyDelete